ConvergePoint vs Xoralia vs DocRead: A Real-World Policy Management Comparison
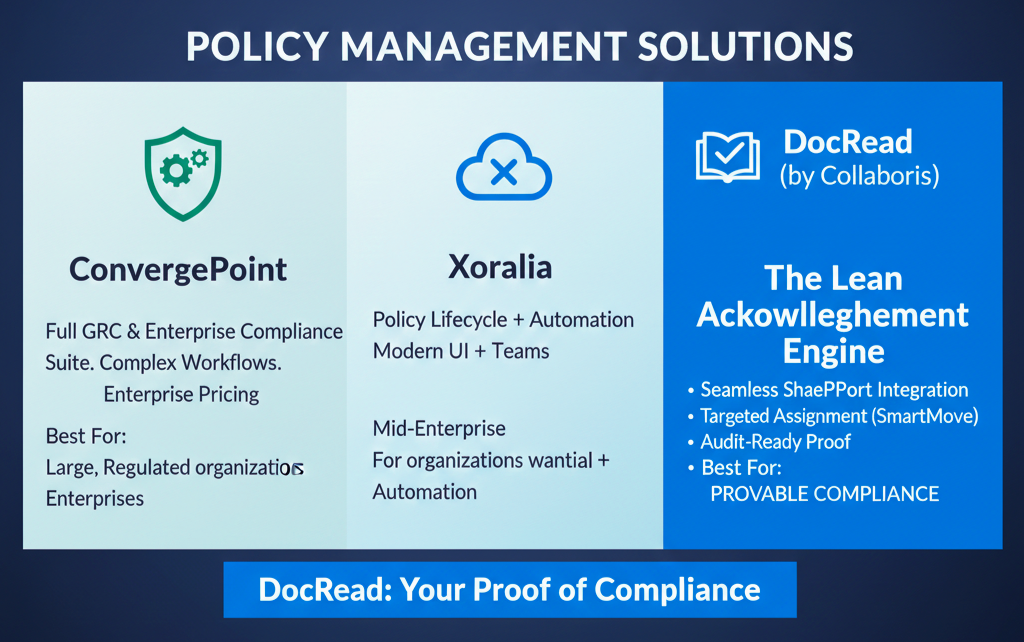
ConvergePoint vs Xoralia vs DocRead: A Real-World Policy Management Comparison If your organization uses Microsoft 365 / SharePoint to manage policies and compliance, picking the right tool matters—especially when proving that people actually read critical documents.ConvergePoint, Xoralia, and DocRead all operate around policy and compliance, but they serve different needs. Below is a straight comparison […]
Read more